Call & WhatsApp: +91-73375-30225
24/7 Email: bonhoahealth@gmail.com
No products
Prices are tax excluded
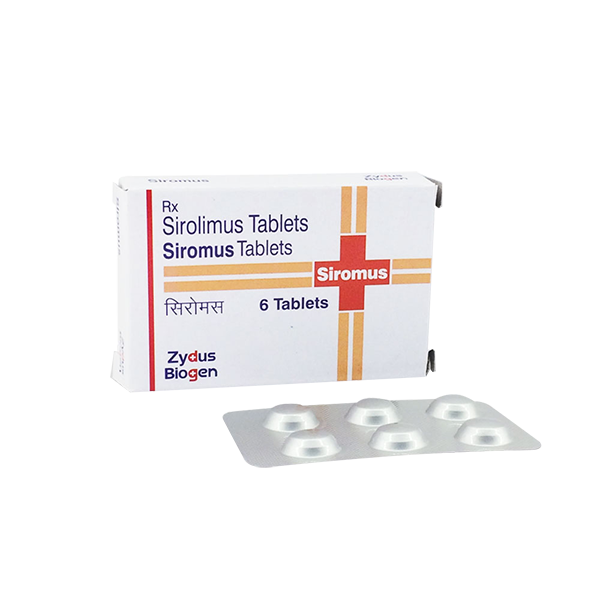 View larger
View larger Siromus(Sirolimus)1mg
Sirolimus
New product
Indications & Dosage
Siromus is also known as Indian Generic Rapamune (Sirolimus), is an mTOR inhibitor immunosuppressant indicated for the prophylaxis of organ rejection in patients aged ≥13 years receiving renal transplants.
Patients at low- to moderate-immunologic risk: Use initially with cyclosporine (CsA) and corticosteroids. CsA withdrawal is recommended 2-4 months after transplantation.
Patients at high-immunologic risk: Use in combination with CsA and corticosteroids for the first 12 months following transplantation. Safety and efficacy of CsA withdrawal has not been established in high risk patients.
Rapamune is an mTOR inhibitor indicated for the treatment of patients with lymphangioleiomyomatosis
DOSAGE AND ADMINISTRATION
Renal Transplant Patients:
Administer once daily by mouth, consistently with or without food Administer the initial dose as soon as possible after transplantation and 4 hours after CsA.
Adjust the Siromus maintenance dose to achieve sirolimus trough concentrations within the target-range.
Hepatic impairment: Reduce maintenance dose in patients with hepatic impairment. In renal transplant patients at low-to moderate-immunologic risk:
Siromus and CsA Combination Therapy: One loading dose of 6 mg on day 1, followed by daily maintenance doses of 2 mg.
Siromus Following CsA Withdrawal: 2-4 months post-transplantation, withdraw CsA over 4-8 weeks. In renal transplant patients at high-immunologic risk:
Siromus and CsA Combination Therapy (for the first 12 months post- transplantation): One loading dose of up to 15 mg on day 1, followed by daily maintenance doses of 5 mg.
Lymphangioleiomyomatosis Patients:
Administer once daily by mouth, consistently with or without food.
Recommended initial Rapamune dose is 2 mg/day.
Adjust the Siromus dose to achieve sirolimus trough concentrations between 5-15 ng/mL.
Hepatic impairment: Reduce maintenance dose in patients with hepatic impairment. Therapeutic drug monitoring is recommended for all patients
ADVERSE REACTIONS
Prophylaxis of organ rejection in patients receiving renal transplants: Most common adverse reactions (incidence ≥ 30%) are peripheral edema, hypertriglyceridemia, hypertension, hypercholesterolemia, creatinine increased, abdominal pain, diarrhea, headache, fever, urinary tract infection, anemia, nausea, arthralgia, pain, and thrombocytopenia.
Lymphangioleiomyomatosis: Most common adverse reactions (incidence ≥ 20%) are stomatitis, diarrhea, abdominal pain, nausea, nasopharyngitis, acne, chest pain, peripheral edema, upper respiratory tract infection, headache, dizziness, myalgia, and hypercholesterolemia
RUG INTERACTIONS
Avoid concomitant use with strong CYP3A4/P-gp inducers or strong CYP3A4/P-gp inhibitors that decrease or increase sirolimus concentrations
Siromus’s brand name in usa is Rapamune by Pfizer. Cassotide’s is a generic prescription medicine from Zydus, which is one of the biggest pharmaceutical company in India.
See full prescribing information for complete list of clinically significant drug interactions
Additional Info
| Package Quantity | 6 |
| Warnings | Don't take the drug without consulting a qualified doctor or physician |
| Suggested use | Follow the doctor's advice |
| Delivery & Returns | 10-20 Days |
Reviews
Accessories
Customers who bought this product also bought:
-
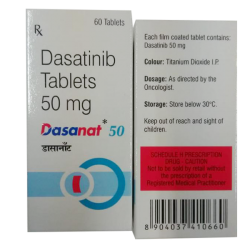
Dasanat (Dasatinib) 50mg
Dasatinib 50mg
$382.00
-
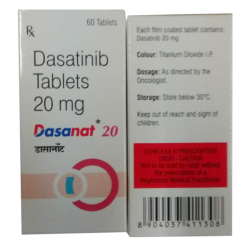
Dasanat (Dasatinib) 20mg
Dasatinib 20mg
$265.00
-

Lucidas (Dasatinib 20mg)
Dasatinib 20mg
$265.00







Thanks bonhoa pharmacy.
I had a kidney transplant in April of 2015 my son was the donor,I was on cellcept and prograf and prednisone, but on July of 2016 I was diagnosed with central nervous system lymphoma,had intense chemo for the last 14 months,they switched me to 2mg.daily use of rapamune with no side effects,my cancer is in remmision and the kidney is doing perfect. Thanks the help of bonhoa pharmacy.